Standardized Mass Cytometry Advances Clinical Decision-Making
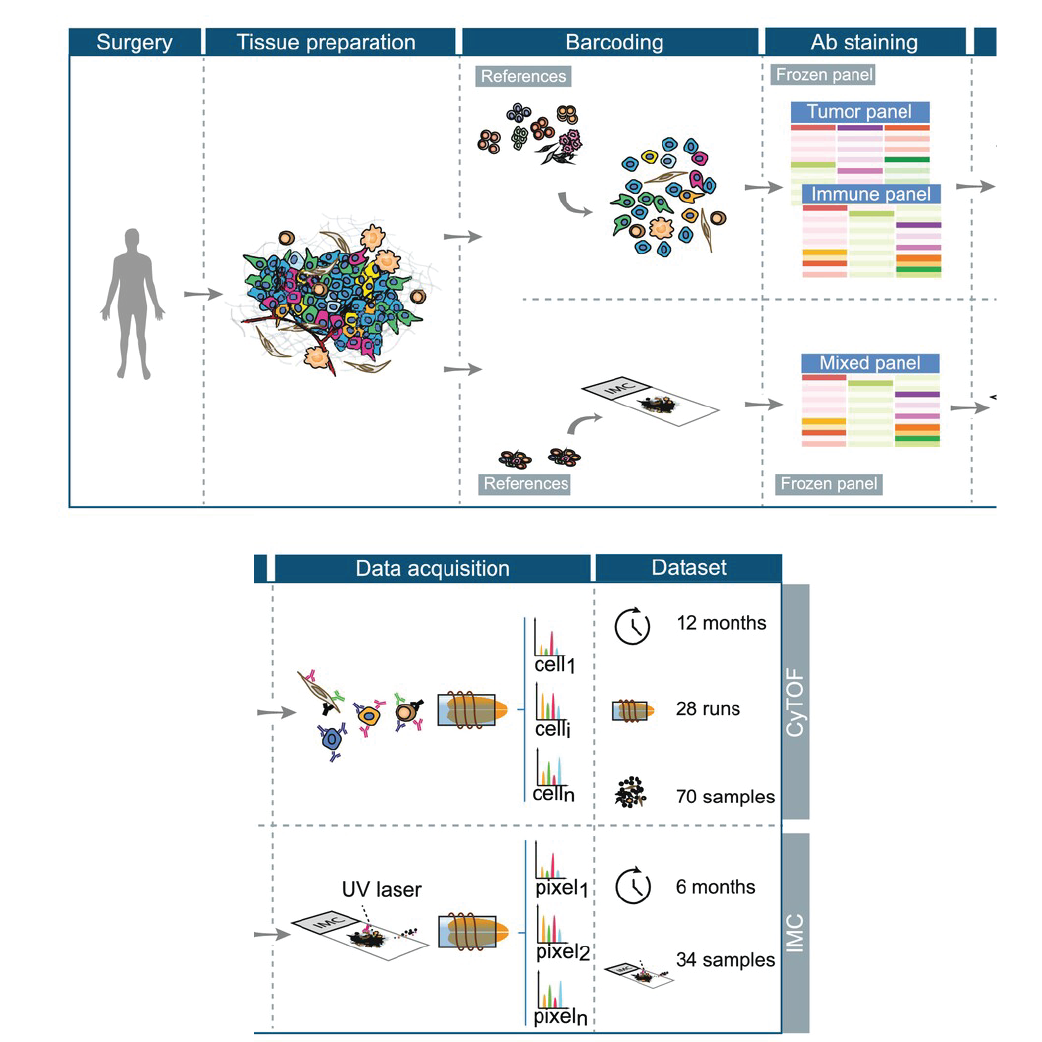
A collaborative study published in Cytometry Part A, involving researchers from the DQBM Bodenmiller Lab and the Tumor Profiler Consortium, has successfully established standardized workflows for suspension and imaging mass cytometry (CyTOF and IMC). These methods significantly enhance the reliability and consistency of single-cell analyses for clinical oncology.
Mass cytometry is powerful for analyzing over 40 markers simultaneously at the single-cell level but previously faced challenges due to technical inconsistencies. To address this, researchers developed rigorous experimental and computational workflows. Innovations included frozen antibody cocktails, robust quality controls, and advanced computational algorithms to correct batch variability.
The new standardized protocols, validated over one year, consistently met clinical standards. The approach was successfully applied in the Tumor Profiler study, accurately characterizing tumor and immune cell populations, thereby aiding personalized clinical decision-making for cancer patients.
This work represents a critical step towards incorporating mass cytometry into clinical practice, enabling precise and personalized patient care.
Publication Link: https://doi.org/10.1002/cyto.a.24940